- KAWAGUTI & PARTNERS Home>
- News Letter>
- February, 2016
K&P’sIntellectual Property High Court Decision Report in 2016
February, 2016
Updated 1 AUG 2016
How should Numerical Values of Peaks in X-ray Powder Diffraction be Construed?
Nissan Chemical Industries, Ltd. v. Sawai Pharmaceutical Co., Ltd., Case No. 2015 (Gyo-Ke) 10081 (Decision rendered on February 24, 2016)
The Patentee, Nissan Chemical, obtained a patent relating to a crystal of a calcium salt of pitavastatin in 2013, against which Sawai, one of the biggest generic drug manufacturers in Japan, filed an invalidation trial with the JPO in 2013. During the trial proceedings, Nissan Chemical demanded a correction of the claims, but the JPO rejected the correction demand and rendered a decision of invalidation in 2015 on the grounds of lacking novelty, inventive step and the like, followed by Nissan Chemical’s appeal against the JPO’s decision to the IPHC in 2015.
Claim 1 of the Nissan Chemical's patent at issue claims as follows:
1. A crystal of a calcium salt of pitavastatin of the following formula (1):
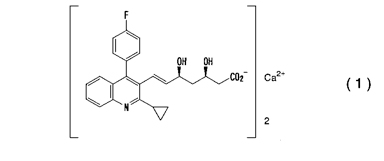
which contains from 7 to 13% of water and which has peaks at a diffraction angle (2q) of 4.96°, 6.72°, 9.08°, 10.40°, 10.88°, 13.20°, 13.60°, 13.96°, 18.32°, 20.68°, 21.52°, 23.64°, 24.12° and 27.00°, and a peak having a relative intensity of more than 25% at a diffraction angle (2q) of 30.16°, considering the peak intensity of diffraction angle (2q) of 20.68° as 100%, in X-ray powder diffraction as measured by using CuKa radiation, provided that the crystal having a melting point of 95°C as measured by differential scanning calorimeter is excluded.
One of the main issues in this case lied in how numerical values of peaks in X-ray powder diffraction should be construed. The IPHC answered to the issue as follows:
In the JPO's decision, the JPO compared the 15 numerical values of the peaks in Claim 1 of the patent at issue and those of the crystal demonstrated in prior art reference (p.a.r.) 3 as below:
| Claim 1 | 4.96 | 6.72 | 9.08 | 10.40 | 10.88 | 13.20 | 13.60 | 13.96 |
|---|---|---|---|---|---|---|---|---|
| p.a.r. 3 | 5.0 | 6.8 | 9.1 | 10.3 | 10.9 | 13.2 | 13.6 | 13.9 |
| Claim 1 | 18.32 | 20.68 | 21.52 | 23.64 | 24.12 | 27.00 | 30.16 |
|---|---|---|---|---|---|---|---|
| p.a.r. 3 | 18.3 | 20.9 | 21.5 | 23.6 | 24.1 | 26.9 | 30.1 |
On the basis of the above comparison, the JPO decided that the crystal in Claim 1 of the patent at issue was substantially the same as the crystal demonstrated in p.a.r. 3 on the grounds that the commentary of 14th Japanese Pharmacopoeia showed a criterion that the same crystal forms would generally conform in the diffraction angles within the range of ±0.2°.
However, having reviewed the description of the specification of the patent at issue and the commentary of 14th Japanese Pharmacopoeia in detail, the IPHC found (i) that the claimed crystal was defined by 15 peaks which were specified by the numerical values with two decimal digits in Claim 1 of the patent at issue, (ii) that no description was found in the specification suggesting (a) that a certain range of margin of error in those numerical values would be allowed or (b) that the claimed crystal form could be identified only by some peaks among the 15 peaks, (iii) that the criterion stated in the commentary of 14th Japanese Pharmacopoeia was the criterion relating to properties and qualities of drugs in healthcare, while it did not apply to numerical values of diffraction angles shown in powder X-ray diffraction measurements in general, and it did not always apply to the examination of the sameness of a patented invention, and (iv) that it had not been technical common knowledge in the examination of the sameness of a patented invention that two crystals could be deemed as identical crystals if numerical values of their diffraction angles were within ±0.2°.
On the basis of the above findings, the IPHC decided that all the 15 claimed peaks in Claim 1 of the patent at issue should be construed as being exactly the same as the numerical values defined therein, thus that Claim 1 of the patent at issue was not the same as the crystal demonstrated in p.a.r. 3.
Conclusively, the IPHC upheld the Nissan Chemical's appeal and cancelled the JPO's decision.
An appeal to the Supreme Court was NOT filed against this decision, and thus the decision is final and binding.
K&P’s CommentsOn the same day this decision was rendered, the same court rendered another decision in an infringement case between Nissan Chemical as the Patentee and Sawai as the accused infringer to the effect that Sawai did not infringe the Nissan Chemical's patent at issue on essentially the same grounds. Namely, in order to satisfy the technical feature of the claimed peaks in Claim 1 of the patent at issue and to find an infringement, it was required that the numerical values of all the 15 peaks of the accused Sawai's crystal exactly conformed to all the claimed numerical values. These two decisions teach that significant figures and allowable measurement errors should be considered very carefully when drafting a specification and a claim relating to a compound defined by the peaks in X-ray powder diffraction.
In February 2016, the IPHC handed down 16 decisions including the above case on patent, and overturned the previous decisions in 4 cases.
In February 2016, the IPHC handed down 3 decisions on trademark, and overturned the previous decisions in 2 cases.



